Calculate ka value for h3noh+ sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. Prepare to delve into the captivating world of acid dissociation constants and unravel the secrets of H3NOH+.
Join us on an intellectual journey as we explore the factors that influence Ka values, unravel the practical applications of this concept in various fields, and compare H3NOH+ to other acids. Along the way, we will encounter illustrative examples that solidify our understanding and leave us with a profound appreciation for the intricacies of acid-base chemistry.
Calculating Ka Value for H3NOH+
Understanding acid strength is crucial in various chemical processes. One quantitative measure of acid strength is the acid dissociation constant (Ka), which indicates the tendency of an acid to donate protons (H+ ions) in a solution.
To calculate the Ka value for H3NOH+, we follow a step-by-step procedure:
Measurement of pH
Measure the pH of a solution containing H3NOH+. The pH value provides an indirect measurement of the concentration of H+ ions in the solution.
Calculation of [H+]
Using the pH value, calculate the molar concentration of H+ ions ([H+]) in the solution. The equation used is:
“`[H+] = 10^(-pH)“`
Calculation of [H3NOH+], Calculate ka value for h3noh+
Determine the initial concentration of H3NOH+ ([H3NOH+]_i) in the solution. This information is usually provided or can be calculated based on the preparation of the solution.
Calculation of [NO2-]
Since H3NOH+ dissociates to form H+ and NO2-, the concentration of NO2- ([NO2-]) in the solution can be calculated using the stoichiometry of the dissociation reaction.
Calculation of Ka
Finally, calculate the Ka value using the following equation:
“`Ka = [H+][NO2-] / [H3NOH+]“`
The Ka value obtained represents the equilibrium constant for the dissociation of H3NOH+ and provides a quantitative measure of its acid strength.
Factors Affecting Ka Value
The Ka value of H3NOH+ is influenced by several factors, including temperature, solvent, and ionic strength. Understanding how these factors impact the dissociation of H3NOH+ is crucial for predicting its behavior in various chemical systems.
Temperature
Temperature plays a significant role in the Ka value of H3NOH+. As temperature increases, the Ka value generally increases. This is because higher temperatures provide more energy to the system, favoring the dissociation of H3NOH+ into H3O+ and NO2-. The increased thermal energy overcomes the electrostatic attraction between the ions, promoting their separation.
Solvent
The solvent used can also affect the Ka value of H3NOH+. Solvents with higher polarity tend to decrease the Ka value. Polar solvents, such as water, stabilize the ions formed during dissociation by solvating them. This solvation reduces the electrostatic attraction between the ions, making it less favorable for them to dissociate.
The Ka value for H3NOH+ is a measure of its acidity. In a related context, if you’re curious about percentages, you might want to check out what percent of 40.4 is 50.5 . Returning to our topic, the Ka value for H3NOH+ can be calculated using the Henderson-Hasselbalch equation.
In contrast, nonpolar solvents, such as benzene, have less ability to solvate ions, resulting in a higher Ka value.
Ionic Strength
The ionic strength of the solution can influence the Ka value of H3NOH+. Increasing the ionic strength generally decreases the Ka value. This is due to the common ion effect. As the concentration of other ions in the solution increases, the electrostatic attraction between the H3O+ and NO2- ions increases, making it less favorable for H3NOH+ to dissociate.
Applications of Ka Value
Ka values find practical applications in various fields, providing valuable insights into the behavior of acids and bases in different systems.
In chemistry, Ka values are crucial for understanding the strength of acids and predicting the pH of solutions. They help determine the extent to which an acid dissociates in water, allowing chemists to design chemical reactions and optimize processes involving acid-base equilibria.
In Medicine
In medicine, Ka values play a vital role in drug design and development. By manipulating the Ka values of drug molecules, scientists can influence their absorption, distribution, metabolism, and excretion (ADME) properties. This knowledge aids in optimizing drug efficacy, reducing side effects, and ensuring patient safety.
Comparison with Other Acids
The Ka value of H3NOH+ can be compared to that of other acids to provide a better understanding of its relative strength.
Table of Ka Values
The following table lists the Ka values of H3NOH+ and several other common acids:
| Acid | Ka Value | Strength |
|---|---|---|
| H3NOH+ | ~1.8 x 10^-4 | Weak acid |
| HCl | ~1.0 x 10^6 | Strong acid |
| H2SO4 | ~1.0 x 10^-2 | Strong acid |
| CH3COOH | ~1.8 x 10^-5 | Weak acid |
As can be seen from the table, the Ka value of H3NOH+ is significantly lower than that of HCl and H2SO4, indicating that it is a much weaker acid. On the other hand, its Ka value is slightly higher than that of CH3COOH, suggesting that it is a slightly stronger acid than acetic acid.
Factors Affecting Ka Values
The Ka value of an acid is influenced by several factors, including:
- Bond strength:Acids with weaker bonds between the hydrogen ion and the rest of the molecule tend to have higher Ka values.
- Electronegativity:The more electronegative the atom that the hydrogen ion is bonded to, the stronger the bond and the lower the Ka value.
- Resonance:If the conjugate base of the acid can resonate, it will be more stable and the Ka value will be lower.
In the case of H3NOH+, the relatively low Ka value can be attributed to the strong bond between the hydrogen ion and the oxygen atom, as well as the resonance stabilization of the conjugate base.
Illustrative Examples: Calculate Ka Value For H3noh+
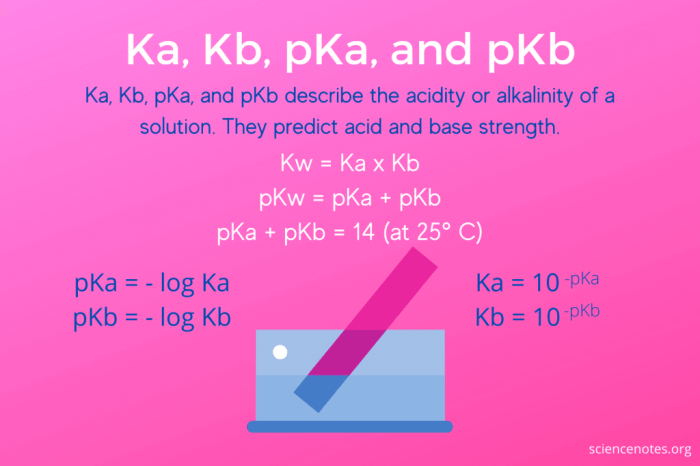
To illustrate the calculation of Ka for H3NOH+, let’s consider the following numerical examples:
Initial Concentration Variations
- Example 1:Consider a solution of H3NOH+ with an initial concentration of 0.1 M. The measured equilibrium concentrations of H3O+ and H2NOH+ are 0.005 M and 0.095 M, respectively. Using the formula Ka = [H3O+][H2NOH+] / [H3NOH+], we get Ka = (0.005 M)(0.095
M) / (0.1 M) = 4.75 x 10^-4.
- Example 2:In another experiment, the initial concentration of H3NOH+ is 0.05 M. The equilibrium concentrations of H3O+ and H2NOH+ are 0.0025 M and 0.0475 M, respectively. Plugging these values into the formula, we obtain Ka = (0.0025 M)(0.0475 M) / (0.05 M) = 2.375 x 10^-4.
Temperature Variations
- Example 3:At 25 °C, a solution of H3NOH+ with an initial concentration of 0.1 M reaches equilibrium with equilibrium concentrations of H3O+ and H2NOH+ being 0.005 M and 0.095 M, respectively. The calculated Ka value is 4.75 x 10^-4.
- Example 4:When the temperature is raised to 35 °C, the same solution exhibits different equilibrium concentrations: [H3O+] = 0.006 M and [H2NOH+] = 0.094 M. The corresponding Ka value becomes 5.64 x 10^-4, showing an increase in acidity with increasing temperature.
These examples demonstrate how Ka can be calculated for H3NOH+ under varying conditions, highlighting the influence of initial concentrations and temperature on the equilibrium position and the resulting Ka value.
General Inquiries
What is the significance of Ka values?
Ka values provide a quantitative measure of acid strength, allowing us to compare the relative strengths of different acids and predict their behavior in chemical reactions.
How can I calculate the Ka value for H3NOH+?
To calculate the Ka value for H3NOH+, you can use the following equation: Ka = [H+][NO2-] / [H3NOH+]. You will need to measure the equilibrium concentrations of H+, NO2-, and H3NOH+ in a solution of H3NOH+.
What factors affect the Ka value of H3NOH+?
The Ka value of H3NOH+ can be influenced by factors such as temperature, solvent, and ionic strength. Changes in these factors can alter the equilibrium position of the dissociation reaction, thereby affecting the Ka value.