Do you need to know solubility rules for mcat – Do you need to know solubility rules for the MCAT? The answer is a resounding yes! Solubility rules are essential for understanding chemical reactions and solutions, and they play a critical role in the MCAT. In this article, we will provide a comprehensive overview of solubility rules, including a list of common exceptions.
We will also discuss how solubility rules are used to predict the solubility of various compounds and how to calculate the solubility product constant (Ksp) for a given compound.
Solubility rules are a set of guidelines that can be used to predict whether a given compound will dissolve in water. These rules are based on the chemical properties of the compound, such as its ionic charge and the size of its ions.
By understanding solubility rules, you can quickly and easily determine whether a compound will dissolve in water, which is a valuable skill for the MCAT.
Solubility Rules for the MCAT: Do You Need To Know Solubility Rules For Mcat
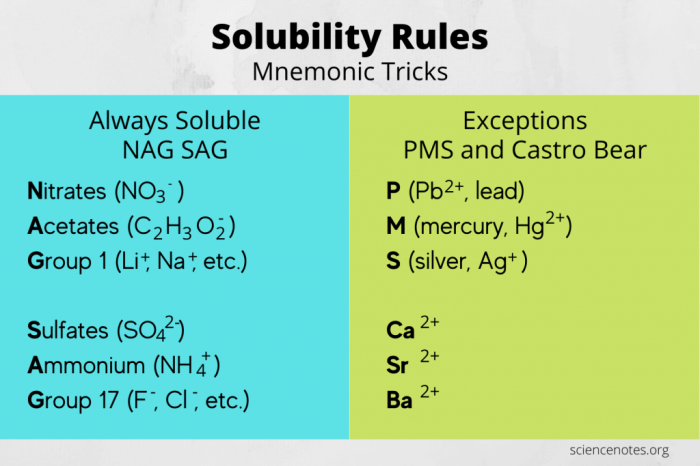
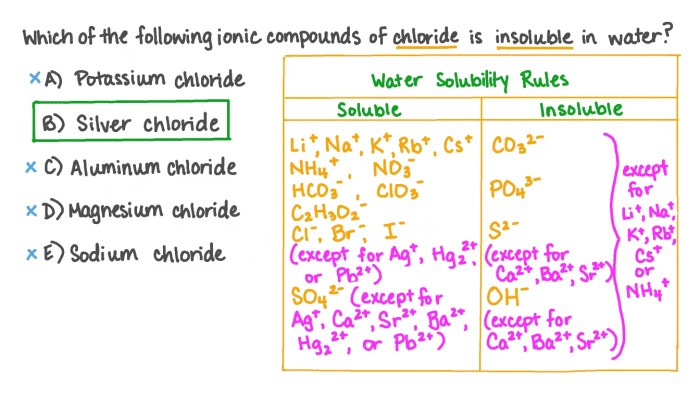
Solubility rules are a set of guidelines that predict the solubility of ionic compounds in water. They are essential for understanding chemical reactions and solutions, and are frequently tested on the MCAT.
Solubility is the ability of a substance to dissolve in a solvent. For ionic compounds, solubility is determined by the interactions between the ions and the water molecules. The solubility rules provide a way to predict these interactions and determine whether a compound will dissolve or not.
Solubility Rules Table, Do you need to know solubility rules for mcat
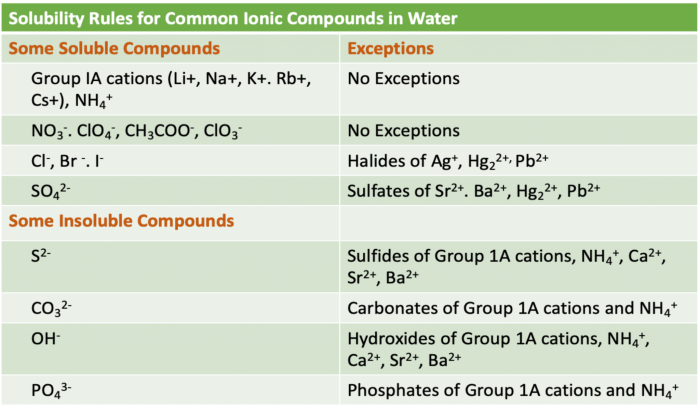
The following table summarizes the solubility rules for ionic compounds:
| Cation | Anion | Solubility |
|---|---|---|
| Group 1 (Li+, Na+, K+, Rb+, Cs+) | All | Soluble |
| Group 2 (Ca2+, Sr2+, Ba2+) | All except sulfate (SO42-) | Soluble |
| Ammonium (NH4+) | All | Soluble |
| Silver (Ag+) | All except chloride (Cl-) | Insoluble |
| Lead (Pb2+) | All except sulfate (SO42-) | Insoluble |
| Mercury (Hg2+) | All except sulfate (SO42-) | Insoluble |
| Copper (Cu2+) | All except sulfate (SO42-), carbonate (CO32-), and hydroxide (OH-) | Insoluble |
| Iron (Fe2+, Fe3+) | All except sulfate (SO42-), carbonate (CO32-), and hydroxide (OH-) | Insoluble |
| Aluminum (Al3+) | All except sulfate (SO42-), carbonate (CO32-), and hydroxide (OH-) | Insoluble |