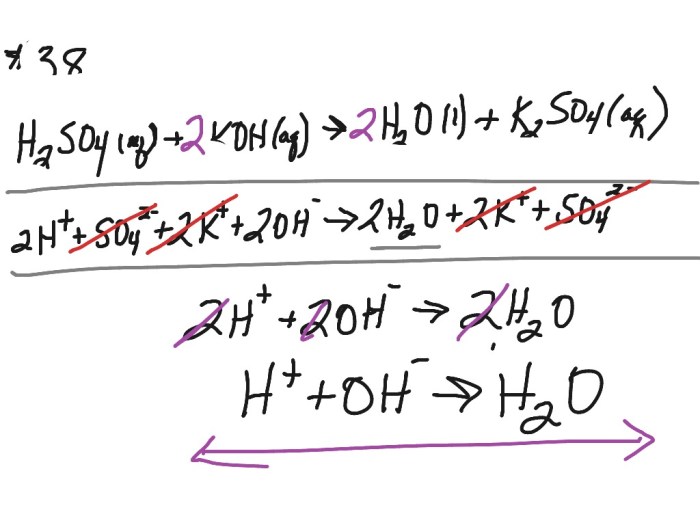Chemistry unit 7 chemical reactions rearranging atoms answer key – Welcome to Chemistry Unit 7: Chemical Reactions Rearranging Atoms Answer Key, where we embark on an exciting journey into the realm of chemical transformations. As we delve into this topic, we will explore the fundamental concept of chemical reactions and how they involve rearranging atoms, leading to the creation of new substances with unique properties.
Throughout this unit, we will uncover the different types of chemical reactions, from synthesis to combustion, and delve into the significance of balancing chemical equations to ensure the conservation of atoms. Along the way, we will encounter practical applications of chemical reactions in various fields, highlighting the power of rearranging atoms to shape our world.
Chemical Reactions: Rearranging Atoms: Chemistry Unit 7 Chemical Reactions Rearranging Atoms Answer Key

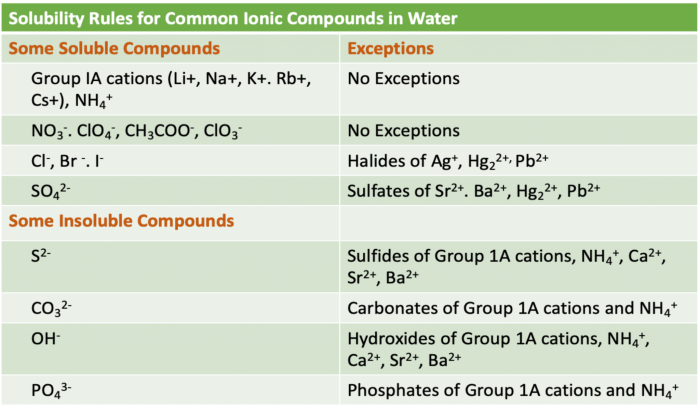
Chemical reactions are processes that involve the rearrangement of atoms to form new substances. Atoms are the fundamental building blocks of matter, and they can be combined in various ways to create an endless variety of molecules and compounds.
When atoms rearrange during a chemical reaction, their electrons are also rearranged. This can lead to changes in the properties of the atoms and the molecules they form. For example, a reaction between hydrogen and oxygen atoms can produce water, which has very different properties than either hydrogen or oxygen.
Types of Chemical Reactions
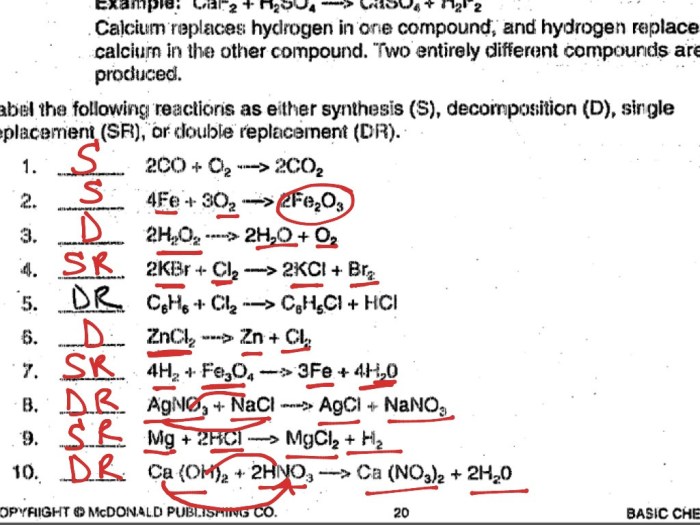
There are many different types of chemical reactions, but they can all be classified into five main categories:
- Synthesis reactions: Two or more substances combine to form a single product.
- Decomposition reactions: A single substance breaks down into two or more products.
- Single-displacement reactions: One element replaces another element in a compound.
- Double-displacement reactions: Two compounds exchange ions to form two new compounds.
- Combustion reactions: A substance reacts with oxygen to produce heat and light.
Each type of reaction involves a specific rearrangement of atoms, and each type has its own unique set of characteristics.
Balancing Chemical Equations
When writing a chemical equation, it is important to balance the equation so that the number of atoms of each element is the same on both sides of the equation. This ensures that the law of conservation of mass is obeyed.
To balance a chemical equation, coefficients are added to the reactants and products. Coefficients are numbers that indicate the number of molecules of each substance that are involved in the reaction. For example, the following equation is balanced:
“`
H2+ O 2→ 2H 2O
“`
This equation is balanced because there are two atoms of hydrogen on both sides of the equation, and two atoms of oxygen on both sides of the equation.
Applications of Chemical Reactions, Chemistry unit 7 chemical reactions rearranging atoms answer key
Chemical reactions are used in a wide variety of applications, including:
- The production of food and beverages
- The manufacture of drugs and other pharmaceuticals
- The production of fuels and other energy sources
- The development of new materials
- The remediation of environmental pollution
Chemical reactions are essential for modern life, and they play a vital role in the functioning of the natural world.
FAQ Resource
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. This adherence to the law of conservation of mass is crucial for accurately representing chemical reactions and predicting the outcome of chemical transformations.
How do chemical reactions contribute to the creation of new substances?
Chemical reactions involve the rearrangement of atoms, leading to the formation of new substances with distinct properties. By breaking and forming chemical bonds, atoms can combine in different ways, resulting in a vast array of molecules with unique characteristics and functionalities.
What are some practical applications of chemical reactions?
Chemical reactions play a vital role in various fields, including medicine, industry, and everyday life. From the synthesis of pharmaceuticals to the production of plastics and fuels, chemical reactions drive technological advancements and shape our modern world.